
BY JOHN COGAN, MD
 |
|
Congestive heart failure (CHF) is a complex syndrome where the ventricle’s ability to fill or eject blood is impaired. It is a final common pathway in a number of conditions that alter the structure or function of the heart. Progression of CHF occurs as a consequence of complex cellular, metabolic, and neurohormonal mechanisms that attempt to compensate for injury.
An estimated five million patients in the United States have CHF, and 550,000 new cases are diagnosed each year, with an annual mortality of 290,000.
Pathology of CHF
The pathophysiology of CHF involves different neurohormonal mechanisms including the sympathetic nervous system (SNS) and the renin-angiotensin-aldosterone system (RAAS). A cascade of effects includes increased afterload and preload, vasoconstriction, sodium and water retention, and cardiovascular remodeling.
The focus of medical therapy is to block neurohormonal compensatory mechanisms that lead to ventricular remodeling and progression of heart failure. Different medications, including ACE inhibitors, angiotensin receptor blockers, spironolactone, beta blockers, digoxin, and diuretics, are used to counteract the neurohormonal compensatory mechanisms.
Ventricular remodeling is defined as a change in the structure and function of the ventricle in response to injury. It includes ventricular dilation, myocyte hypertrophy, interstitial fibrosis, apoptosis, and beta receptor down-regulation.
Cardiac resynchronization therapy
Cardiac resynchronization therapy (CRT) may benefit patients with CHF caused by depressed ejection fraction and who continue to have severe symptoms despite drug therapy.
Between 35 percent and 50 percent of patients with severe CHF have delayed ventricular conduction (wide QRS), which is a marker for ventricular dyssynchrony. These patients have disorganized ventricular activation due to altered electrical conduction, often resulting in inefficient timing of contraction between the septal and lateral walls of the left ventricle (LV). Features of ventricular dyssynchrony include abnormal interventricular septal wall motion, reduction in stroke volume, diminished diastolic filling times, worsening of mitral regurgitation, and compromised pumping effectiveness resulting in decreased cardiac output, reduced ejection fraction (EF), and increased symptoms. Ventricular dyssynchrony can even be seen among patients with systolic CHF and normal QRS duration.
Biventricular devices were designed to treat dyssynchrony. These involve placing three pacing leads, one in the right atrium to synchronize the ventricular conduction with the atria, one in the right ventricle, and a third lead inserted via the coronary sinus to a postero-lateral branch of the left ventricle (LV). With this, atrial contraction is synchronized to ventricular contraction as with a regular pacemaker. Additionally, by adjusting the timing of the right and left ventricular activation and contraction, the left ventricular systolic function may be resynchronized. This results in increased LV systolic function, which thereby can reduce symptoms of CHF and improve patient outcomes.
Several randomized trials have shown evidence that CRT improves patient symptoms, hemodynamics, functional status, quality of life, and ventricular remodeling and reduces hospitalizations. The recent Cardiac Resynchronization in Heart Failure (CARE-HF) trial demonstrated that CRT not only improves symptoms, but also decreases mortality.
When implanting a CRT-defibrillator, not only do patients benefit from the effects of alleviating dyssynchrony, but also from having a backup implantable defibrillator. This includes the ability to overdrive pace or shock dangerous ventricular arrhythmias and has proven in many randomized trials to help reduce mortality.
CRT is indicated in patients with medically refractory CHF with persistent NYHA class III or IV symptoms with a QRS wider than 120 msec (evidence of dyssynchrony) and an ejection fraction equal or below 35 percent.



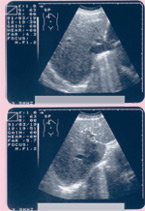 If there is clear dyssynchrony, physicians recommend resynchronization as part of the strategy of treatment for these patients. Based on the practice’s previous experience, and consistent with reports from other literature, patients with borderline or normal QRS width, but with echo-proven dyssynchrony, have excellent response to CRT.
If there is clear dyssynchrony, physicians recommend resynchronization as part of the strategy of treatment for these patients. Based on the practice’s previous experience, and consistent with reports from other literature, patients with borderline or normal QRS width, but with echo-proven dyssynchrony, have excellent response to CRT.