BY DANIEL NORBERG, MD, FACC
 |
|
Congestive heart failure (CHF) is a complex clinical syndrome with multiple etiologies. The signs and symptoms of CHF are secondary to the body adapting through various mechanisms to a dysfunctional heart. The congestion of body tissues occurs as the body attempts to maintain a steady state and preserve function.
CHF accounts for millions of hospitalizations per year and is the most common patient discharge diagnosis in the United States. It afflicts 1% to 2% of the U.S. population with the prevalence nearly doubling for each decade of life. Nearly one-third of the presenting CHF population has normal systolic function with the predominance of symptoms secondary to diastolic dysfunction. The treatment of cardiac disease and the population’s advancing age contribute to the increases in the incidence and prevalence of CHF. Because intervention and aggressive therapy lead to decreased symptom progression and mortality, early recognition is crucial.
Structural dysfunction
 |
|
The heart is often described as a sophisticated pump that delivers blood to the body. Cardiac output refers to the dynamic circulation throughout the body per minute and is dependent on the overall function of the heart and many factors controlling cardiac performance. The body can manipulate cardiac output through a complex system of checks and balances. Ultimately, the goal of each organ system (brain, heart, kidneys, etc.) is to preserve perfusion for survival. If the heart is functioning at less than maximum efficiency, organ systems recognize decreased perfusion and send messages to the heart to increases cardiac output. As the heart becomes more dysfunctional, the body, compensating for decreased cardiac output, contributes to further physiologic decompensation and worsened cardiac function. This vicious cycle, although simplistic, illustrates the syndrome of CHF.
There are two components of heart failure involving the impairment of systole (passive phase) and diastole (energy consumption phase) of the cardiac cycle. Systolic dysfunction refers to the heart’s decreased ability to contract and provide forward ejection of blood into the circulation. Diastolic dysfunction refers to a decreased ability of the heart to relax and fill with blood prior to contraction. These types of dysfunction may exist separately or together. As much as 30% of the heart failure population has combined systolic and diastolic dysfunction. This combination complicates the medical therapy and requires close monitoring of responses to treatment modalities.
Left heart failure is the functional decline of the left ventricle and makes up the majority of the population of heart failure patients. As the right heart function declines, signs and symptoms of right heart failure follow. Right and left heart failure occur separately or together. Typically, left heart failure precedes right heart failure. As the poorly functioning left ventricle fails, the blood pressure within the lungs increases. Unable to generate pressures to match that seen within the lungs, the right ventricle fails. Occasionally, right heart failure occurs with normal left ventricular function (pulmonary hypertension, tricuspid, or pulmonic valvular disease). Cardiomyopathy refers to changes in the structure of the heart muscle in response to impaired function.
Common etiologies
There are many etiologies of cardiomyopathy and cardiac dysfunction. Common etiologies include ischemic (coronary artery disease, myocardial infarction), hypertension, postviral syndrome, restrictive (i.e., amyloidosis), hypertophic, valvular dysfunction, pulmonary hypertension, metabolic (thyroid disorders, mineral deficiencies, alcohol), and idiopathic. These disease processes lead to decreased cardiac performance and circulatory system changes. These changes become evident in a patient’s decreased general well-being and physical condition. The presentation and progression of CHF progress are variable, and the signs and symptoms are related to conditioning and the severity of the failing heart chambers.
In left heart dysfunction, there are symptoms of dyspnea with exercise and/or at rest, orthopnea, paroxysmal nocturnal dyspnea, fatigue, decreased functional capacity, anorexia, and nocturia. Signs include weight gain, evidence of increased pulmonary markings on a chest roentgenogram, interstitial pulmonary edema and pleural effusion, hypoxia, and pericardial effusion. Symptoms of right heart failure may include some shortness of breath or fatigue. The signs of right ventricular failure include lower extremity edema, change in abdominal girth associated with ascites, jugular venous distention, and weight gain. Once heart failure is suspected, diagnostic testing is necessary to determine the etiology and severity of heart failure.
Evaluation via left heart catheterization is necessary to rule out the possibility of coronary artery disease, measure left heart pressures, and evaluate left ventricular function. Right heart catheterization measures the pressure within the right side of the heart and pulmonary arteries and estimates the left ventricular filling pressure. If suspected, incorporation of endomyocardial biopsy with right heart catheterization can identify a myocardial disorder. Stress testing with cardiac imaging allows for evaluation of ischemia, cardiac perfusion, and cardiac function. Transthoracic echocardiogram is useful in the evaluation of left and right ventricular function, wall motion abnormalities, and valvular dysfunction.
When there is insufficient data from transthoracic echocardiography, a transesophageal echocardiogram provides a clearer view of the heart. Once cardiomyopathy is diagnosed and the etiology investigated, initiation of therapy for CHF is essential.
Patient, help thyself
The most important component to the success in the treatment of heart failure is the compliance of the patient with his or her diet, fluid restriction, exercise, and medical therapy. Patient education and involvement in the treatment protocols are crucial. Changes in diet are necessary and essential. Patients with heart failure need to be aware of sodium consumption — namely the use of table salt and natural sodium contents in the foods consumed. It is important to understand that wherever salt goes, water will follow. Increased salt consumption in conjunction with increased fluid consumption leads to fluid retention. The typical low-sodium diet consists of less than 2 grams per day.
Fluid restriction makes up the second component of patient involvement in decreasing fluid retention. Thirst increases as the heart fails and occurs in response to decreased cardiac output and the body thinking it is depleted of fluid. Frequently, there is too much fluid in the circulatory system, which leads to increased work for the heart, edema, pulmonary edema, and/or pleural effusion, ascites, or pericardial effusion.
Medication therapies
Medications have become the hallmark for treatment of CHF. Current aggressive medical regiments increase functional capacity, decrease mortality, and increase the well-being of the patient. Currently, medical therapy encompasses afterload reducers, preload reducers, ionotropic agents, chronotropic agents, and peptides. These various medications together provide the greatest amount of symptom relief and improved longevity. The treatment of systolic dysfunction is well studied and provides important guidelines in regard to medical therapy. Therapy for diastolic dysfunction is anecdotal and based in theory.
Currently, the uses of angiotensin-converting enzyme inhibitors (ACE inhibitors) or the combination of hydralazine and isosorbide dinitrate are effective afterload-reducing medications. Angiotensin receptor blockers are approved for treatment of CHF; ACE inhibitor therapy, however, remains the treatment of choice. Studies have shown the use of afterload-reducing medications slows disease progression, decreases symptom progression, and decreases mortality. These medications are available in an intravenous and oral form. Intravenous milrinone and dobutamine also are effective afterload-reducing medications, but they are not beneficial for long-term therapy. The use of afterload-reduction medication requires careful observation when used in combination with preload reduction medication.
The normal heart can increase contraction based on the amount of stretch that occurs in diastole or ventricular relaxation. In the dysfunctional heart, however, this reflex does not respond appropriately, and the pressures within the heart increase. This increased pressure transmits into the lungs and venous system, causing edema. To decrease the filling volumes and pressures, medications such as long-acting nitroglycerin and diuretics are used to decrease preload.
It is important to mention that a certain amount of elevation in preload is needed in the dysfunctional heart, especially when there is diastolic dysfunction. Decreasing preload through diet, fluid restriction, and sodium restriction are important in the management of CHF. Overall, the control of afterload and preload is a delicate balance in a failing heart.
 |
|
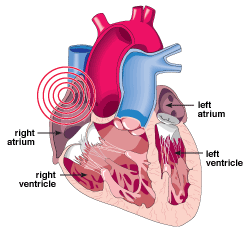
| TThe sinus node is found in the right atrium, where the electrical pulse that makes the heart beat begins. An irregularity in these pulses, such as when the heart beats faster or slower than normal, is known as an arrhythmia. |
For some time, medications to slow the heart rate were used, primarily in the treatment of diastolic dysfunction and heart failure, to increase ventricular filling time and relaxation. Beta-blockers are beneficial in the treatment of left ventricular systolic dysfunction, diastolic dysfunction, and heart failure. The use of beta-receptor blocker therapy in heart failure revolves around the theory that, as the heart fails, the levels of catecholamine increase in the circulation.
Elevated catecholamine levels lead to increased heart rate and force of heart contraction, leading to an increase in cardiac output. Chronic elevations of catecholamines in the bloodstream are toxic to the heart muscle and can cause further worsening of function. The use of beta-receptor blocker therapy decreases the response of the heart to the elevated catecholamine levels. Studies have shown that the use of this medication, in conjunction with current medical therapy for CHF, increases functional capacity, decreases the need for hospitalizations, and decreases mortality. Despite negative chronotropic effect, the use of calcium channel blocker therapy has no role in the treatment of systolic dysfunction secondary to negative ionotropic effect. The negative ionotropic effect has shown some benefit in increasing diastolic filling times and improving symptoms associated with diastolic dysfunction.
With the exception of digoxin, intravenous medications in this class require a monitored environment, such as a hospital or special heart failure clinic. Digoxin therapy has no advantage for isolated diastolic dysfunction and could have a deleterious effect in patients. Direct physician and nursing observation is necessary with close monitoring of heart rate, heart rhythm, and blood pressure. Currently, intravenous ionotropic medications, such as dobutamine, milrinone, and dopamine, are short-term therapy secondary to the poor outcomes realized with prolonged infusion.
Overall, these medications are effective in the improvement of cardiac function and well-being of the patient when used in short-term support. Despite improvements in a patient’s functional capacity on milrinone and dobutamine, long-term follow-up studies suggest decreased patient longevity on these medications. Digoxin is the only oral medication proven safe for long-term ionotropic support. The use of intravenous ionotropic therapy has fallen out of favor in the face of newer peptide therapy.
Human B-type natriuretic peptide is secreted by the heart as part of the body’s normal response to heart failure. The intravenous use of nesiritide is helpful in decreasing dyspnea symptoms of CHF by decreasing preload and reducing pulmonary capillary wedge. The medication is only approved for use in a monitored hospital setting. To date, this drug has never been shown in a controlled trial to reduce mortality in CHF patients.
Patients with CHF due to diminished systolic function are at increased risk for dying suddenly from ventricular arrhythmias. It has now been demonstrated that implantable defibrillators can reduce sudden cardiac death and overall mortality in patients with CHF and decreased ejection fraction. Furthermore, in patients with wide QRS complexes and decreased ejection fraction, the use of biventricular pacing to resynchronize left ventricular function has been shown to improve symptoms of CHF and overall cardiac function in a majority of patients indicated for this procedure.
More drastic measures
In the event that the patient remains unresponsive to medical therapy, mechanical and surgical support are available. The uses of an intra-aortic balloon pump and a biventricular assist device are available in the short term and experimentally in the long-term treatment of CHF. Currently, the intra-aortic balloon pump temporarily assists the heart in an aggressive effort to treat heart failure unresponsive to medical therapy alone. The use of a biventricular assist device is as a bridge to cardiac transplantation in a patient who fits the strict criteria.
Overall, important strides have been made toward improving functional capacity, decreasing symptoms, and decreasing mortality of patients with heart failure. The overlap of systolic and diastolic ventricular dysfunction continues to pose challenges to CHF management.


