BY JUAN PASTOR-CERVANTES, MD, FACC
 |
|
My interest in extracranial carotid disease spans nearly 10 years, and it began at a time when carotid endarterectomy (CEA) indication for patients with symptomatic lesions showed a clear benefit over conventional medical therapy. Since that time, minimally invasive therapies like carotid stenting have emerged as viable alternatives for patients who are deemed at high risk for surgery or poor candidates for CEA, which is considered the standard of care.1
Stroke is the most common life threatening neurologic disease and the third leading cause of death in the United States, exceeded only by heart disease and cancer. It is responsible for one of every 15 deaths. Despite being more often disabling than lethal, 167,660 deaths were attributed to stroke in 2000.2 That same year, the American Heart Association estimated that there were 500,000 initial strokes, 200,000 stroke recurrences, and 4,700,000 stroke survivors in the United States, many of whom required chronic care.3 It is estimated that by 2015, the elderly population (>65 years) will increase to 14% of the population, up from 2.3% in 1900. This growth in the elderly population will be a source of continuing disability from stroke unless vigorous and effective preventive measures are implemented more aggressively.
Stroke prevalence
Overall, the annual age-adjusted (ages 35 to 94 years) total initial completed stroke event rates were 5.9/1,000 in men and 4.9/1,000 in women — a 20% excess rate is observed in men. The annual age-adjusted incidence of isolated transient ischemic attack also increases with age and was 1.2/1,000 in men and 0.71/1,000 in women.4
Stroke registries and prospective population studies indicate that there is a disproportionate rate of ischemic stroke occurrence after awakening between 6 a.m. and noon.5 An increased rate of intracerebral hemorrhage was reported in men who drink, possibly reflecting binge drinking on the weekend.6
More than three million Americans have symptomatic cerebrovascular disease. Its prevalence is much higher in blacks (2.6%) than in whites (1.6%) for patients between the ages of 25 and 74.7
The short-term, intermediate, and long-term adjusted mortality rates were investigated in the Department of Veterans Affairs care system in veterans who had experienced initial hemorrhagic or ischemic strokes. These investigators found a 30-day mortality rate of 7.4% and 18.8% for ischemic and hemorrhagic strokes, respectively. The adjusted 90-day mortality rate for ischemic stroke was 11.4%, and, for one year, 19.1%. The adjusted mortality rate for 30-day survivors of a hemorrhagic stroke was 5.6% for 30 to 90 days and 7.3% for three months to one year.8
The frequency of completed strokes by type was nearly identical in men and women. Atherothrombotic brain infarction (including infarction secondary to large vessel atherothrombosis, lacunar infarction, and infarct of undetermined cause) accounted for more than 61.5% of strokes in men and 60% in women. Extracranial large artery disease accounted for approximately 10% to 20% of cases of cerebral infarction. Intracranial hemorrhage accounted for 14% of completed strokes in men and 13.4% in women.
Risk factors for stroke
A number of modifiable atherogenic stroke risk factors were identified from The Framingham Study and other prospective epidemiological studies. These risk factors include elevated blood pressure, diabetes mellitus, obesity, hyperlipidemia, elevated blood homocysteine levels, impaired left ventricular systolic function, coronary artery disease, atrial fibrillation, left ventricular hypertrophy, migraine, intracranial aneurysms, sleep apnea, cigarette smoking, oral contraceptive use, and alcohol consumption.
Extracranial carotid artery disease
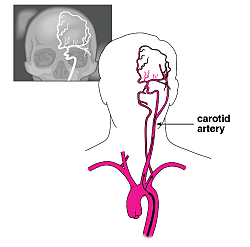 |
|
| Atrial fibrillation can cause a clot to leave the heart and lodge in the brain, resulting in a stroke. This cerebral arteriography shows possible paths from the heart to the brain. |
Carotid atherosclerosis develops in areas of low vessel wall shear stress, most commonly the carotid bulb.
The earliest lesion of atherosclerosis is the fatty streak, which is an infiltration of monocyte-derived macrophages and T-lymphocytes in the arterial wall. Fatty streaks occur early in life, involving the aorta in the first decade of life and the coronary and extracranial carotid arteries in the second decade. The fatty streaks start as an infiltration of low-density lipoprotein (LDL) cholesterol in the arterial wall, followed by its oxidation. The process continues when macrophages secrete chemokines and mitogens that induce smooth muscle cell proliferation. This progression may lead to plaque growth and eventual narrowing of the vessel lumen.
In the carotid bifurcation, atherosclerosis is most severe in the posterior wall of the carotid bulb, where there is low shear stress and greater turbulence. Disturbed laminar flow in the carotid bulb may lead to high adhesion molecule expression of endothelin, among other changes. Most myocardial infarctions are associated with thrombosis in plaques with inflammatory cell content and large necrotic lipid cores, so-called unstable plaques. This mechanism has not been established as clearly in the carotid artery, although recent evidence suggests that occlusion of the extracranial carotid artery bifurcation has a similar pathophysiology.9,10
Approach to the patient
The first and most important approach to the patient with carotid atherosclerosis is to initiate strategies to modify vascular risk factors and stabilize and prevent the progression of carotid atherosclerotic plaque. Therefore, aggressive blood pressure reduction in hypertensive patients is essential. Management of hyperlipidemia, obesity, diabetes, obesity control, smoking cessation counseling, and lifestyle-changing strategies are all of extreme importance.
The second approach is the initiation of antithrombotic therapy. Strong evidence supports the use of antiplatelet therapy for secondary stroke prevention. All available anti-platelet agents have demonstrated benefits in reducing recurrent stroke rates. The choice between aspirin, clopidogrel, ticlopidine, or a combination and modified-release dipyridamole will depend on the patient’s risk-factor profile, side effects, and cost.
Warfarin is not superior to aspirin in reducing recurrence of noncardioembolic ischemic events and probably should not be used routinely in this population because of its potential hemorrhagic complications.11 A possible exception is the intraluminal thrombus in the internal carotid artery (ICA). These patients are managed preferably with anticoagulation for a few weeks, followed by CEA.12
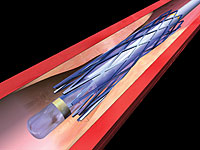
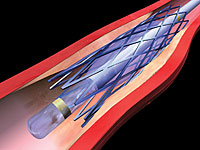
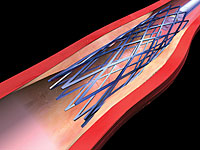
The third approach is the removal of the atherosclerotic plaque through surgery or an endovascular procedure, known as carotid artery stenting, or CAS (see Figure 1).
Carotid artery revascularization
Surgical treatment for carotid stenoses was introduced in the early 1950s. Prospective observational data suggested benefit for surgery over medical therapy, but large prospective randomized trials investigating the effect of CEA on stroke reduction were not completed until the late 1980s and early 1990s.
Studies such as the North American Symptomatic Carotid Endarterectomy Trial (NASCET),13 the European Carotid Surgery Trial (ECST), and the Asymptomatic Carotid Atherosclerosis Study (ACAS) confirmed the short- and long-term superiority of surgery over medical management in symptomatic and asymptomatic patients with significant carotid stenosis.
The CEA studies excluded patients who were at high risk for this surgery. Thus, patients were excluded if they had previous ipsilateral endarterectomy, intracranial stenosis that was more severe than the surgically accessible lesion, unstable angina pectoris, recent myocardial infarction, recent contralateral CEA, progressive neurologic dysfunction, recent surgical procedure, long-term anticoagulation therapy, and surgically inaccessible lesions.14
Cardiovascular complications of surgery, such as myocardial infarction and congestive heart failure, are well recognized, and a significant number of patients were reported to have transient myocardial ischemia and occult infarction associated with adverse cardiac events.15
CEA has significant surgical complications not always reported (as was the case with the ESCT study) but should be acknowledged. In the NASCET, perioperative wound complications (9.3%) and cranial nerve damage (8.6%) emphasized the adverse events associated with an open surgical procedure16. Medical complications were common (8.1%), largely of cardiovascular origin (7.1%), including myocardial infarction (1.2%) and congestive heart failure (1.2%).16
The mission to develop safer, percutaneous solutions to carotid arterial stenosis was pioneered in 1977 by Mathias and colleagues, who performed the first balloon angioplasty to the carotid artery bifurcation.17
In 1986, Rabkin18 reported the use of primitive nitinol stents in the carotid artery, and Theron19 began pivotal work with distal balloon protection during carotid bifurcation angioplasty. In 1994, Roubin and his group at the University of Alabama began routine carotid stenting under rigorous institutional protocols.20 Later, the use of distal protection devices was recognized as an integral part of carotid artery dilatation and stenting.21 The high technical success rate noted in the early works and the progress made over the years have led to an exponential growth in the number of cases around the world.22
The state of carotid stenting
The indications for intervention with carotid stenting are similar to those of carotid surgery with respect to symptomatic status and severity of carotid stenosis. This approach to patient management is based on documented, prospective outcome assessment from many experienced operators and centers. Many operators are now providing credible outcome data to support such an approach. In intracenter analysis, if prospective, independent neurologic audit shows, for example, that in a group of surgically eligible symptomatic or asymptomatic patients, the 30-day death rate is less than 1% and total 30-day stroke events are less than 3%, then stenting should be an attractive therapeutic option.
There are notable exceptions to these therapeutic principles. Subsets of patients are emerging as better candidates for stenting, and other patients are better candidates for CEA. It is, therefore, now becoming important for the medical community to understand the relative indications and contraindications. Patients who have comorbid medical conditions that increase their risk from an open surgical approach or the use of general anesthesia are one important group that should be primary candidates for stenting. Based on the results of the recently published Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy (SAPPHiRE) and ACCULINK for Revascularization of Carotids in High-Risk Patients (ARCHeR) trial, those conditions include advanced age (>80 years), New York Heart Association class III/IV for congestive heart failure, chronic obstructive pulmonary disease, contralateral occlusion, prior CEA, prior myocardial infarction within 30 days, unstable angina, two or more coronary vessels with more than 70% stenosis, a need for coronary artery bypass graft or valve surgery within 30 days, dialysis dependant-renal patient, or unfavorable anatomy (i.e., s/p radical neck surgery, prior neck radiation, surgically inaccessible lesions, spinal immobility, tracheostomy stoma, contralateral laryngeal nerve paralysis).23
However, until recently the carotid stent trails have focused on indications for high-risk patient populations that are unlikely to answer the overall question of whether carotid artery stenting with state-of-the-art technology (embolic protection devices) is equivalent to the standard of care (i.e., CEA) for most patients with carotid stenosis who are at risk for stroke.
The Carotid Revascularization using Endarterectomy or Stenting Systems (CaRESS) trial was designed on the basis of the broad category of standard risk by using symptomatology as a defining criterion for stratification in the study.
The choice of treatment by CAS or CEA was based solely on physician and patient preference. This design more accurately reflects the true clinical environment. The 30-day composite morbidity and mortality results of 4.4% for CEA and 2.1% for CAS compare well with both NASCET and ACAS. The cumulative one-year major adverse event rates in SAPPHiRE were 20.1% for CEA and 12.2% for CAS; those in CaRESS were 14.3% vs. 10.9%, respectively.
The CaRESS phase 1 study suggests that the risk of death or stroke one year after CAS by using distal protection is equivalent to that after CEA in a broad-category population with carotid stenosis. The CaRESS phase 1 study was able to closely resemble clinical practice by enrolling patients on the basis of the degree of carotid stenosis and symptomatology rather than surgical risk.24
Conclusion
Stroke is a major threat to our aging patient population for whom surgical options are not always well-tolerated or accepted. Certainly, it will take a few more years to know if minimally invasive therapies like carotid stenting indeed will replace carotid surgery as the choice of treatment, as it has done with other vascular territories.
So far, it can be clearly stated that carotid stenting with distal protection, by experienced operators, is the procedure of choice for high-surgical-risk patients with extracranial carotid disease for stroke prevention and that carotid artery stenting is likely the treatment of choice for low-risk surgery patients in the near future.
References
- Zarins CK. Carotid endarterectomy: the gold standard. J Endovasc Surg 1996; 3:10–15.
- American Heart Association. Heart and Stroke Facts. Dallas 1994.
- Sacco RL, Ellenberg JH, Mohr JP, et al. Infarct of undetermined cause: the NINCDS stroke data bank. Ann Neurol 1989;25:382.
- Sacco RL, Wolf PA, Kannel WB, et al. Survival and recurrence following stroke. The Framingham Study. Stroke 1982;13:290.
- Marler JR, Price JR, Clark GL, et al. Morning increase in onset of ischemic stroke. Stroke 1989;20:473.
- Kelly-Hayes M, Wolf PA, Kase CS, et al. Temporal patterns of stroke onset: The Framingham Study: Stroke 1995;26:1343.
- NHLBI, NHANES 1971-1994 public date release file documentation. National Center for Health Statistics, 1999.
- Collins TC, Petersen NJ, Menke TJ, et al. Short-term, intermediate-term, and long-term mortality in patients hospitalized for stroke. J Clin Epidemiol 2003;56.
- Garcia JH, Khang-Loon H. Carotid atherosclerosis, definition, pathogenesis and clinical significance. Neuroimaging Clin N Am 1996;6:801–10.
- Fischer CM. Occlusion of the internal carotid artery. Arch Neurol Psychiatr 1951; 65:346–377.
- Mohr JP, Thompson JL, Lazar B, et al. A comparison of warfarin and aspirin for the prevention of recurrent ischemic stroke. N Engl J Med 2001; 345:1441–51.
- Buchan A, Gates P, Pelz D, Barnett HJM. Intraluminal thrombus in the cerebral circulation: implications for surgical management. Stroke 1988;19:681–98.
- North American Symptomatic Carotid Endarterectomy Trial collaborators: Beneficial effect of carotid endarterectomy in symptomatic patients with high grade carotid stenosis. N Engl J Med 1991;325:445.
- National Institute of Neurological Disorders, and Stroke and Trauma Division. North American Symptomatic Carotid Endarterectomy Trial (NASCET) Investigators. Clinical alert: benefit of carotid endarterectomy for patients with high-grade stenosis of the internal carotid artery. Stroke 1991;22: 816.
- Landersberg G, Shatz V, Akopnik I, et al. Association of cardiac troponin, CK MB, and postoperative ischemia with long term survival after major vascular surgery. J Am Coll Cardiol 2003;42:1547.
- Paciaroni M, Eliasziw M, Kappelle LJ, et al. Medical complications associated with carotid endarterectomy: North American Symptomatic Carotid Endarterectomy Trial (NASCET). Stroke 1999;30:1759.
- Mathias K: Ein Neuariges Katheter-system zur perkutanen transluminen angioplastic von Karotiss stenosen. Fort Schr Med 1977;95:1007.
- Rabkin I, Germashev VG. Five year experience with roentgenologically controlled endovascular nitinol prosthesis. Kardiologia 1990;30:11.
- Theron J, Raymond J, Casasco A; et al. Percutaneous angioplasty of atherosclerotic and postsurgical stenosis of carotid arteries. AJNR Am J Neuroradiol 1990;11:869.
- Yadav JS, Roubin GS, Iyer S, et al. Elective stenting of the extracranial carotid arteries. Circulation 1997;95:376.
- Iyer S, Roubin GS, Vitek J, et al. Carotid artery stenting with neuroprotection. Circulation 2002;39:30A.
- Wholey MH, Wholey M, Mathias K, et al. Global Experience in cervical carotid artery stenting placement. Catheter Cardiovascular Interventions 2000;50:160.
- Yadav J, Ouriel K: Impact of medical vs. anatomic risk factors on 30 day and 1 year outcomes in the SAPPHiRE Trial. Circulation 2003;108: IV–603.
- CaRESS Steering Committee. J of Vasc Sur. Vol 42, No.2, 213–219.